After concerns about the health impacts of BPA emerged, some efforts were made to remove BPA from consumer products. Unfortunately, in many cases, BPA was substituted with other bisphenols that are now linked to similar health harms.
Chemical Structure
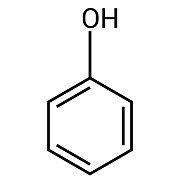
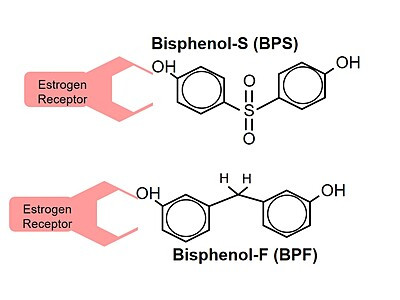
As the name bisphenol suggests, bisphenols are made up of two phenol groups. A phenol group is a hydroxyl (―OH) group attached to an aromatic ring, as shown in the following structure diagram.1
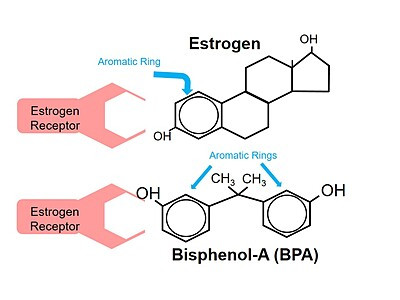
As shown below, estrogen also has a phenol group. For estrogen, the phenol group is a core structure for attachment to estrogen receptors.
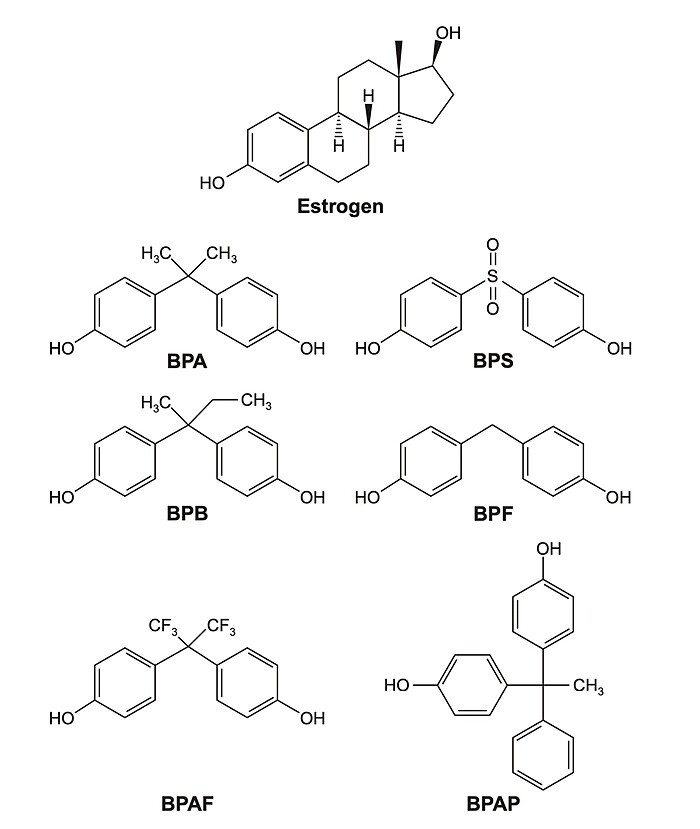
The same is true for BPA. BPA mimics estrogen, binding to estrogen receptors.3 While it is not known yet if all bisphenols mimic estrogen in the same way, the structural similarities make it likely that many bisphenols also bind to estrogen receptors.4 As a result, they can be expected to have similar health impacts. In fact, research has been showing for years that these BPA-substitute bisphenols can have similar endocrine-disrupting effects.5
Health Impacts
BPA was the first bisphenol to be manufactured on a large scale, with commercial production starting in the 1950s.7 Because of its early widespread use, BPA is the most well-studied of the bisphenols.8 Studies conducted in the 1930s indicated BPA to be estrogenic.9 However, it would be some time before the risks of BPA would be more fully understood.
Endocrine Disruption
Today, BPA is known to be a potent endocrine disrupting chemical (EDC). EDCs can interfere with the body’s endocrine system and produce adverse developmental, reproductive, neurological, and immune effects in both humans and wildlife. In addition to being estrogenic, BPA can bind to androgen receptor (AR), thyroid hormone receptors (TRα and β), estrogen-related receptor gamma (ERRγ), and glucocorticoid receptor. All of these interactions could contribute to adverse health impacts.
BPA is a major risk factor for endocrine, immune, and oncological diseases.10 As one review article summarized, “low doses of BPA alter hormone-sensitive organs and are related to a wide range of human noncommunicable diseases.”11
The most vulnerable groups are fetuses and children, because the effects of EDCs during critical developmental periods are irreversible. Possible diseases and disorders from exposures during critical windows include these:12
- Prostate cancer
- Breast cancer
- Uro-genital abnormalities in male babies
- A decline in semen quality in men
- Early onset of puberty in girls
- Metabolic disorders including type 2 diabetes and obesity
- Neurobehavioral problems such as attention deficit hyperactivity disorder
The table below shows a more complete list of suspected health impacts of BPA. Unless otherwise noted, the information in the table is from a 2020 review of the health impacts of BPA.13 As previously discussed, BPA is the most well-studied bisphenol, but similarities in the chemical structures make it likely that other bisphenols will have similar impacts.
|
Health Outcome |
Observed in laboratory animals |
Associations in epidemiology studies |
|
Metabolic disease: |
|
|
|
Obesity |
√ |
√ |
|
Impaired glucose tolerance |
√ |
√ |
|
Type 2 diabetes |
√ |
√ |
|
Hypertension |
√ |
√ |
|
Cardiovascular disease/dysfunction |
√ |
√ |
|
Altered liver function |
√ |
√ |
|
Adrenal hyperplasia |
√ |
|
|
Altered thyroid function14 |
√ |
|
|
Neural and behavioral effects: |
|
|
|
Sex-specific changes in brain structure |
√ |
|
|
Altered estrogen action |
√ |
|
|
Aggression/aggressive behaviors |
√ |
√ |
|
Impaired learning/memory |
√ |
√ |
|
Male reproduction: |
|
|
|
Reduced libido |
|
√ |
|
Altered sperm count, sperm quality |
√ |
√ |
|
Abnormalities of spermatogonia |
√ |
|
|
Urethra–bladder obstruction and hydronephrosis |
√ |
|
|
Uro-genital abnormalities15 |
|
√ |
|
Reduced serum testosterone |
√ |
|
|
Prostate hyperplasia, metaplasia, neoplasia |
√ |
|
|
Female reproduction: |
|
|
|
Uterine fibroids |
|
√ |
|
Ovarian cysts, polycystic ovarian syndrome |
√ |
√ |
|
Chromosomal abnormalities, oocytes |
√ |
|
|
Early onset of puberty |
√ |
√ |
|
Mammary gland development, hyperplasia |
√ |
|
|
Mammary cancer |
√ |
|
|
Estrous cycle/menstrual cycle disruption |
√ |
|
|
Reduced serum estradiol |
√ |
|
|
Risk of miscarriage |
|
√ |
|
Preterm birth16 |
|
√ |
|
Immunological effects:17 |
|
|
|
Oxidative stress |
√ |
|
|
Decreased regulatory T cells |
√ |
|
BPA, like other EDCs, has been shown to have a non-linear dose-response relationship in some situations. In non-linear dose-responses, a higher dose/exposure level does not necessarily equate to a stronger effect. For instance, a toxicant might have a stronger effect at low and high doses, but show a lesser effect at medium doses.
BPA has been shown to have a non-linear impact on estrogen levels during pregnancy.18 These non-linear relationships make risk assessment more complex, which can delay regulatory steps to protect health.
A 2015 review concluded that BPA replacements may have similar health effects as BPA,19 and a 2016 review of hormonal activities of BPS and BPF found their potency to be in the same order of magnitude and of similar action as BPA.20
Exposure Sources
Despite efforts to remove BPA from products, production has increased from an estimated 7.69 million metric tons in 2015 to 9.2 million metric tons in 2023. This is estimated to reach 12 million metric tons by 2032.21 These estimates don’t include any of the replacement bisphenols now being produced.
Bisphenols are widely used in polycarbonate plastics and epoxy resins. They can be used in plastic products like medical devices, toys, food containers, eating utensils, and cups. Epoxy resins made from bisphenols are routinely used as linings for food containers, including canned foods and beverages.
Some products that are commonly made with bisphenols include:22
 Canned food and drinks
Canned food and drinks- Baby bottles and nursing products
- Dental sealants and orthodontic products
- CDs and DVDs
- Eyeglasses
- Medical equipment and tubing
- Consumer electronics
- PVC
- Plasticizers
- Water bottles and other food and beverage containers
- Canned food and drinks
- Water supply pipes
- Thermal paper (receipts)
- Impact-resistant safety equipment, such as sports safety equipment
Bisphenols readily leach out of their products, contaminating food, water, and air. Bisphenols are now omnipresent contaminants in the environment. As a result, we are all chronically exposed to bisphenols. Bisphenols can enter our bodies through touch, inhalation, and consumption of contaminated foods.23
For most people, the primary source of exposure is from dietary exposure, including consumption of food contaminated in the environment and food and drink contaminated by food packaging materials.
The second most important source of exposure is through absorption through the skin, from sources including thermal paper (receipts), toys, medical devices, and other plastics. The third most important sources of exposure is inhalation, from the air and bisphenol-contaminated dust.24
A 2008 study measured BPA levels in the urine of nearly 3,000 randomly selected people (six years old and above), with BPA found in over 92 percent of the people tested. Since then, BPA has been detected in human placentas,25 amniotic fluid, maternal and fetal plasma,26 and breast milk.27
Reducing Exposures
While individuals can and should take personal steps to avoid bisphenols, it is not realistic or equitable for society to handle bisphenols in this way. With bisphenols used in so many consumer products, they are largely unavoidable. This is why we need stronger regulations that can protect everyone’s health.
Regulation
In 2015, the European Food Safety Authority (EFSA) assessed the safe limit for daily intake of BPA to be 4 μg/kg body weight (bw). In 2023, EFSA completed a new assessment based on more recent studies using animal data and human observational studies. Effects from BPA on the immune system were identified at low doses. Based on that, the assessed safe limit for daily intake of BPA was lowered to 0.2 ng/kg bw. This new safe limit is 20,000 lower than the previous limit.28 As a result of this new assessment, the European Union is moving to ban the use of BPA in food contact materials.29 The ban will also apply to several BPA replacements, including BPS.
In contrast, the Food and Drug Administration (FDA) has banned the use of BPA in only a limited number of products, such as in baby bottles and sippy cups. Otherwise, FDA still allows the widespread use of BPA in food containers and packaging. The allowable estimated daily intake is 500 ng/kg bw, thousands of times more than what EFSA considers safe.30
Some states are taking more proactive measures than FDA. At least thirteen states and the District of Columbia have enacted their own restrictions on BPA since 2009. These restrictions variously involve cups and beverage containers, especially those intended for young children; all children's products; pacifiers; infant formula packaging; sports bottles; thermoses; all food containers; and thermal receipt paper.31
Unfortunately, most of these regulations do not address the regrettable substitution of BPA with other bisphenols. For instance, FDA has no provisions for regulating bisphenols as a class. Here, some states are taking the lead. In 2019, Washington state passed the Safer Products for Washington Act. The act regulates dangerous classes of chemicals, so that removing one problematic chemical doesn’t simply result in it being replaced by a similar chemical. The act regulates bisphenols as a class.
FDA could follow Washington’s lead and enact similar protective measures. Until that happens, the regrettable substitution of one bisphenol for another will continue.
Limiting Personal Exposures
- Use glass food and beverage storage containers.
- Use a reusable glass or stainless steel bottle for drinking water.
- Avoid heating plastic in a microwave, as heating can increase the rate of leaching or degrade the plastic.32
- Avoid handling cash register receipts.
- When you can, choose fresh whole fruits and vegetables.33
 Many products now claim to be “BPA-free,” but unfortunately that designation is effectively meaningless. In many cases, the BPA was simply substituted with other harmful bisphenols.34
Many products now claim to be “BPA-free,” but unfortunately that designation is effectively meaningless. In many cases, the BPA was simply substituted with other harmful bisphenols.34
This page was last revised in October 2024 by CHE’s Science Writer Matt Lilley with input from Haleigh Cavalier, and editing support from CHE Director Kristin Schafer.
Some information on this page is sourced from Toxipedia.
CHE invites our partners to submit corrections and clarifications to this page. Please include links to research to support your submissions through the comment form on our Contact page.