People long ago recognized that, depending on the dose, arsenic could either treat an illness or be used as a poison to cause death. Its medicinal use to treat syphilis and amoebic dysentery ended with the introduction of penicillin and other antibiotics in the twentieth century. Arsenic-based compounds are currently used to treat some forms of cancer. As a poison, arsenic trioxide (As2O3) looks like sugar, is tasteless, and only a fraction of a gram can kill an adult.
Chemical & Biological Properties
|
|
Arsenic is a versatile metal, forming various compounds, either inorganic or organic.
Inorganic forms of arsenic (such as arsenic trioxide) are much more toxic than organic forms. Inorganic arsenic is widely distributed in nature. Most rocks contain one to five parts per million (ppm) of arsenic.
Organic arsenic is produced in a biomethylation process by many organisms including humans and shellfish. Organic arsenic compounds found in seafood are not well absorbed.
In contrast, soluble inorganic arsenic compounds, such as arsenic trioxide, are readily absorbed from the intestine (80-90%). Arsenic can also be absorbed through the lungs and skin. Most of the arsenic in the blood is bound to red blood cells.
Once ingested, inorganic arsenic is biotransformed by the liver to a methylated form of arsenic and excreted in the urine with a half-life of three to five days. Arsenic is also excreted in the outer layer of skin cells and in sweat. Arsenic also concentrates in the hair and fingernails.
Health Impacts
Acute exposure
Ingestion of 100 to 300 mg of arsenic trioxide can be fatal.1 A 100-mg potentially lethal dose is the same as 0.1 grams.
Initial effects from acute poisoning may be delayed for several hours. Acute oral ingestion of lower doses can result in these symptoms:2
-
Irritation of the stomach and intestines, with symptoms such as stomachache, nausea, vomiting, and diarrhea.
- Decreased production of red and white blood cells, which may cause fatigue, abnormal heart rhythm, blood-vessel damage resulting in bruising, and impaired nerve function causing a "pins and needles" sensation in the hands and feet.
- White bands, called Mees' lines, are visible in the nails.3
Chronic exposure
The International Agency for Research on Cancer (IARC), the US Department of Health and Human Services (DHHS), and the US EPA have all classified arsenic as a cause of cancer.4
Chronic arsenic poisoning (arsenicosis) is most often associated with drinking water contaminated with arsenic. Signs and possible impacts of arsenic exposure:5
- Skin cancer
- Cancer of the liver, bladder, kidney, or lungs
- Skin changes including patches of darkened skin and the appearance of small "corns" or "warts" on the palms, soles, and torso; these are often associated with changes in the blood vessels of the skin.
- Possibly diabetes, high blood pressure, reproductive disorders, and lower IQ scores
Inhaling high levels of inorganic arsenic is associated with these signs and symptoms:6
- Sore throat and irritated lungs
- Skin effects described above
- Circulatory and peripheral nervous disorders
Direct skin contact with high concentrations of inorganic arsenic compounds may cause the skin to become irritated, with some redness and swelling.7
Much less is known about the health effects of organic arsenic compounds (such as methyl and dimethyl compounds). In animals, ingestion of methyl compounds can result in diarrhea, and lifetime exposure can damage the kidneys. Lifetime exposure to dimethyl compounds can damage the urinary bladder and the kidneys.8
Vulnerable populations
Children have higher levels of exposure to arsenic, particularly if drinking water concentrations are elevated, because of their smaller size and greater consumption of water relative to their size. Children also play in soil, put their hands in their mouths, and intentionally eat soil more often than adults do. Exposures in childhood also afford many more years for long-latency diseases and disorders (such as cancer) to develop than adult exposures.
Workers in industries that involve arsenic production or use can be exposed to elevated levels of arsenic. Those who saw wood treated with chromated copper arsenate (CCA), plus all people who live near or frequent mining and smelting sites or agricultural areas where arsenic pesticides had been applied in the past, may be exposed to much higher levels of arsenic than others.9 In addition, arsenic is used in the manufacture of silicon-based computer chip technology10 and in glass manufacture.11 Elemental arsenic is used as an alloying element in ammunition and solders, as an antifriction additive to metals used for bearings, and to strengthen lead-acid storage battery grids.
Exposure Sources
Background exposures
Most people are exposed to constant but low levels of arsenic. Normally, background air levels are less than 0.1 μg/m3 and drinking water less than 5 μg/L, but water levels can be significantly higher. The total average daily exposure to arsenic is about 20 μg/day from food and water (assuming 2000 mL/day average water consumption at 5 μg/L arsenic), but this can vary significantly depending on diet and water source.
Water exposure
Contaminated groundwater presents the greatest threat to public health from arsenic.12 Arsenic poisoning from well water remains a serious human health concern worldwide. This has been particularly devastating in Bangladesh, where tens of millions of people are estimated to have been exposed to arsenic-contaminated water, resulting in an estimated 43,000 deaths each year.13 In Argentina, Chile, and Taiwan, elevated arsenic in drinking water has been associated with increases in mortality from internal cancers.14 Worldwide, over 100 countries are affected by groundwater arsenic contamination.15
In the United States, about seven percent of the wells sampled contained arsenic at a concentration that exceeded 10 µg/L.16 The map below shows estimates of how many private domestic well users in each county may be drinking water with elevated levels of arsenic.
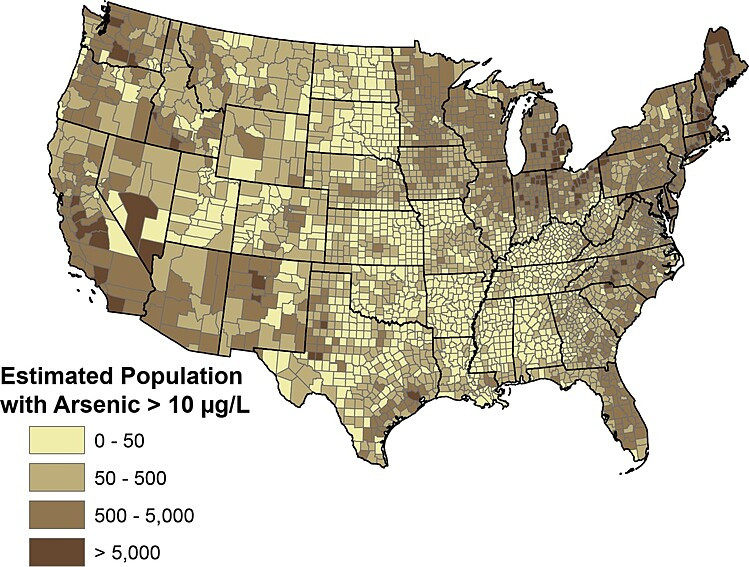
An estimated 2.1 million people in the U.S. may be drinking domestic well water that is high in arsenic.18
Climate change is expected to exacerbate already existing issues with arsenic in well water. In Bangladesh, sea level rise from climate change is expected to decrease the concentration of oxygen in groundwater, as well as increase the salinity. Both of these changes would result in increased arsenic concentrations.19
In addition to sea level rise, climate change can modify other groundwater conditions (including temperature, recharge, storage, flow, and water-table depth). Individually or in combination, these changes could potentially lead to increased arsenic levels through altered hydrodynamics, enhanced water–rock interactions, and modified hydrogeochemical environments.20 For example, reductions in groundwater recharge (water moving down from the surface into aquifers) attributed to climate change in the Mediterranean region have led to increased arsenic in soils and shallow aquifers.21
Food & agriculture
Arsenic may be present in grains, fruits, vegetables, and other foods. Arsenic exposure from ingested foods usually comes from food crops grown in arsenic-contaminated soil and/or irrigated with arsenic-contaminated water.22 Most crops don’t readily take up much arsenic from the ground, but rice is an exception—it takes up arsenic from soil and water more readily than other grains.23 In addition, some seafood has high levels of less toxic organic arsenic. Food usually supplies less than 10 μg/day of arsenic but can be higher with the consumption of rice and fish, and particularly shellfish, which can have arsenic levels up to 30 μg/g.
The US Food and Drug Administration (FDA) has been monitoring the levels of arsenic in foods for decades and increased its testing in 2011. Arsenic levels in apples have been raised as a concern, but FDA's results have confirmed that the amount of arsenic in apple juice is generally low.24
Inorganic arsenic had been used as a pesticide in cotton fields and orchards, although that use was voluntarily canceled in the late 1980s and the early 1990s.25 Some forms of organic arsenic are applied to cotton fields as herbicides. Until recently, organic arsenic compounds were also used as a feed additive to enhance growth of poultry and swine.
Pressure-treated wood
|
|
The major use of inorganic arsenic has been in wood preservation, although its use for residential wood preservation has been phased out.26 Wood treated with the pesticide chromated copper arsenate (CCA) was first used in the 1940s. CCA is a water-based mixture of inorganic salts of chromium, copper, and arsenic that is forced into wood under pressure. Arsenic can transfer to hands by direct contact. Children who play on decks or other treated surfaces pick up small amounts of arsenic on their hands, from which it can be transferred to food or directly from hands into children's mouths. The potential exposure to children from playing in structures constructed from CCA-treated wood is generally smaller than what they would receive from food and water.27
The amount of arsenic in treated wood can be quite large. A standard eight-foot length of treated 2" x 4" lumber contains as much as 15 grams of arsenic. Inhaling sawdust from construction with treated lumber is known to be dangerous.28 Arsenic leaches from the wood with rainfall, and contamination of soil under decks may exceed hazardous waste cleanup standards.29
Wood treated with CCA is still found in decks, playground equipment, outdoor furniture, fences, construction lumber, utility poles, piers, and pilings. In December 2003, manufacturers voluntarily discontinued manufacturing CCA-treated wood products for homeowner uses. However, EPA has not banned CCA and does not require the removal of existing structures made with CCA-treated wood or the surrounding soil.
There are a number of arsenic-free wood preservatives on the market that are registered for use in treated wood for residential use. CCA is still available for commercial uses such as utility poles. The alternative wood treatment most used to replace CCA is a copper-based preservative called ammoniacal copper quaternary ( ACQ). ACQ has a much lower toxicity to humans than CCA.30
Smelting
Arsenic is produced as byproduct of smelting for copper, lead, and zinc. The last US smelter producing arsenic closed in 1985 in Tacoma, Washington. Smelters typically released arsenic trioxide and lead into the atmosphere, which contaminated local environments and left an unhealthful legacy for local residents. Inorganic arsenic is also released from coal-fired electric generation facilities.
Since US production ceased in 1985, all arsenic consumed in the United States is imported. Most of the arsenic imported to the US comes from China and Morocco.31 In 2005, 11,000 metric tons of arsenic trioxide was imported into the United States.32
Tobacco
Cigarettes contain small amounts of arsenic, and smokers inhale some arsenic in tobacco smoke.33
Reducing Exposures
Regulatory standards
Government agencies routinely test public water supplies for arsenic. In contrast, property owners are usually responsible for testing private well water (see below). The FDA tests food and bottled water in the United States for arsenic. The FDA's goal is to limit consumer exposure to arsenic, with a focus on protecting the very young, through developing regulations, action levels, and providing educational information.
- World Health Organization: provisional drinking water guideline of 10 μg/L (10 ppb)34
- US EPA: Drinking water 10 μg/L (10 ppb)35
- OSHA: no greater than 10 micrograms of inorganic arsenic per cubic meter of air (10 µg/m3), averaged over any eight-hour period for a 40-hour workweek36
- EPA Reference Dose (RfD) for inorganic arsenic is 0.3 μg/kg/day, based on hyperpigmentation, keratosis, and possible vascular complications in humans. The RfD is an estimate that is likely to be without appreciable risk of deleterious noncancer effects during a lifetime.37
Personal prevention
Because contaminated groundwater is the most common source of arsenic exposure,38 it is important to test well water for arsenic. New wells should be tested at least once. Over time, arsenic levels in a well water can change. Therefore, it is recommended that wells be periodically retested. States issue guidelines for recommended well testing schedules for arsenic (and other contaminants). Owners of private wells should contact their state drinking water well program if a problem is suspected. If arsenic is detected, either a different drinking water source should be used or the water should be treated to remove the arsenic.39
Taking these additional steps can also help prevent arsenic exposure:
- Avoid inhalation of sawdust from arsenic-treated lumber, and never burn any treated lumber or sawdust.
- If you have an older deck or other structure made with CCA-treated wood, applying a penetrating protective coating (such as oil- or water-based stains) on a regular basis may reduce the leaching of chemicals.40
- Testing the soil underneath decks and other structures made with treated wood will indicate whether remediation of soil is needed.
- Wear gloves and other protective clothing when working with treated lumber or contaminated soil.
- Wash your hands after coming in contact with any arsenic-treated product, and supervise children in washing their hands promptly after contact.
- Take care to reduce arsenic exposure from rice, especially for children or if you consume more rice than average. In 2014 Consumer Reports investigated which varieties of rice have higher levels, cooking methods that reduce arsenic levels, and alternate grains that are naturally lower in arsenic.41
This page was last revised in March 2025 by CHE’s Science Writer Matt Lilley, with editing support from CHE Director Kristin Schafer.
![]() Some information on this page is sourced from Toxipedia.
Some information on this page is sourced from Toxipedia.
CHE invites our partners to submit corrections and clarifications to this page. Please include links to research to support your submissions through the comment form on our Contact page.



